NICA STANDARDS DEVELOPMENT
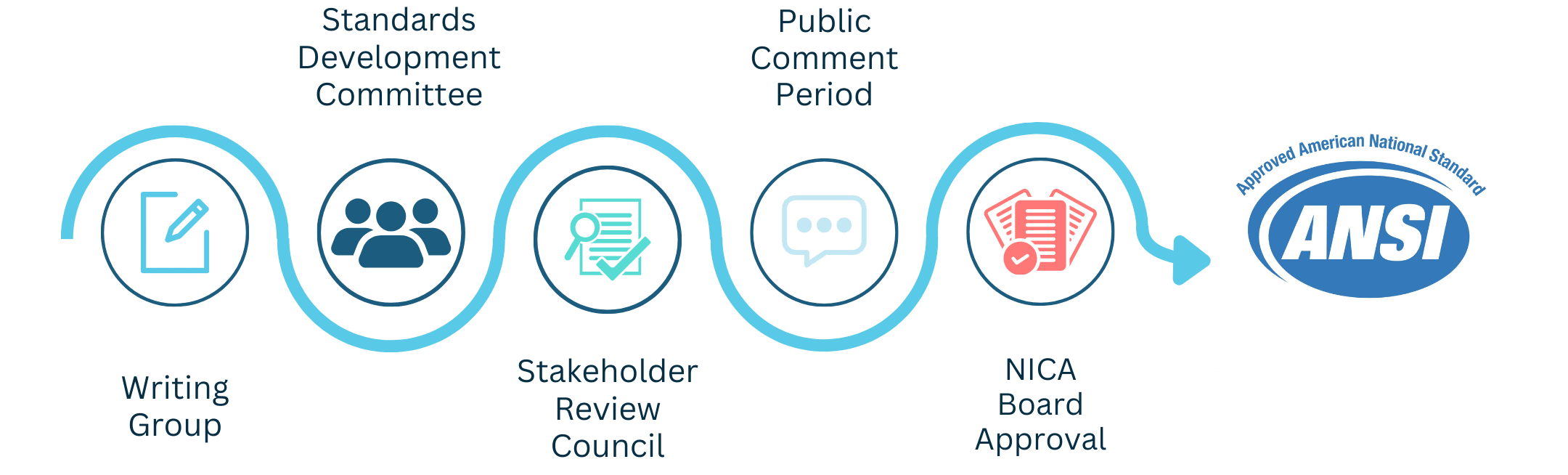
Step 1: Development
-
The Standards Development Committee drafts proposed standards.
-
Draft circulates among the Standards Development Committee for feedback and revisions.
-
This iterative process is repeated as many times as needed to achieve consensus.
Step 2: Council Review
- The proposed standards are then circulated to a larger group of industry stakeholders called the Stakeholder Review Council for feedback.
- Committee reviews feedback, and edits and recirculates the draft if needed.
- When the initial draft is complete, Committee members vote to approve the proposed standards.
Step 3: Public Review
- The Committee-approved standards undergo a defined public review period where members of the public are able to provide feedback.
- Public comments are considered and responded to, with every effort made to ensure relevant objections and comments are addressed appropriately.
- If a revision is needed, the draft goes back to the Committee (Step 1).
- If revisions are not needed and there are no deliberations or appeals, NICA Board reviews documentation for procedural compliance.
Step 4: ANSI Submission
- Following NICA Board approval, the proposed standards—along with meticulous documentation of every vote, comment, objection, and so forth—are sent to the ANSI Executive Standards Council.
Step 5: ANSI Approval
- ANSI reviews all of the documentation to ensure procedural requirements are met.
- The standards are approved as American National Standards.
Standards Development Committee
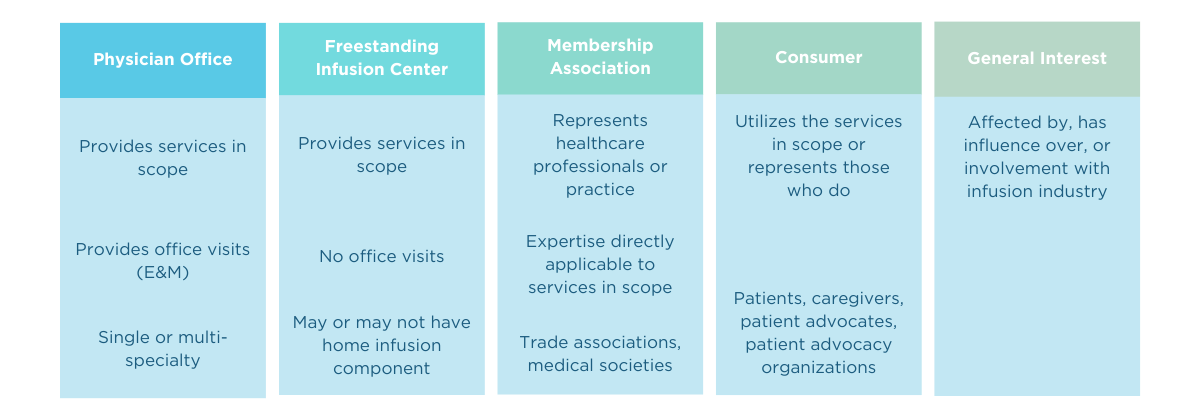
In order to ensure a balanced representation of stakeholders, applicants were divided into “interest categories” based on the nature of their classes of trade and/or relationship to the infusion industry.
NICA took extra steps to ensure a balance of not just interest categories, but also specialties and professions.
STANDARDS DEVELOPMENT COMMITTEE
STAKEHOLDER REVIEW COUNCIL
Official Consensus Body; Drafts and Approves Standards
Rating Group; Reviews Draft Standards and Provides Feedback
Voting Members
Non-Voting Members
Members Appointed by the Board of Directors
Stakeholders with Direct/Material Interest; Possess Relevant Professional Experience
Knowledgable About the Subject Matter; Directly Impacted by the Standard(s)
12-18 Members
15+ Members
Single 'Interest Group" May Not Contribute to More than 1/3 of Membership
No Limitations on Size or Composition for the Stakeholder Review Council
2025-2026 Standards Development Committee Members
Editor-in-Chief
Kaitey Morgan, BSN, RN, CRNI
Chief Clinical Officer, National Infusion Center Association (NICA)
NICA Organizational Representative
Chair
Edie Gigot. MSN, RN, MBA
Chief Operating Officer, AdvisorWoRx
STANDARDS DEVELOPMENT MEMBERS
Physician Office-Based Infusion Center
VP of Quality & Patient Safety, Articularis Healthcare Group
Melissa Mangieri, RNClinical Director for Bendfusion, Bendcare
Cheryl VaughnVP of Clinical Services, Intrafusion by McKesson
Karen McKerihan, NP-CInfusion Director, Low Country Rheumatology
Jennifer McClain, RNDirector of Clinical Excellence, Corinthian Health Services
Freestanding Infusion Center
Compliance Officer, Twelve Stone Health Partners
Andra Tisdale, PharmDDirector of Clinical Programs and Quality Assurance, Palmetto Infusion Services
Fara Stricker, MSN, RN, FNP-CChief Nursing Officer, ThrIVewell Infusion
Rebekkah JackmanVP of Clinical Operations, FlexCare Infusion Centers
Erica Smith, RN, IgCNDirector of Nursing Services, Intramed Plus
Consumer
Executive Director, Infusion Access Foundation
Danielle Pietraszewski, RDHRegistered Dental Hygienis
General Interest
Partner, Leech Tishman
Edie Gigot, RN, MSN, MBAChief Executive Officer, AdvisorWoRx, LLC
Membership Association (organizational representatives)
American Gastroenterological Association Joseph Feuerstein, MD, ADAF
Associate Professor,
Harvard Medical School
Beth Israel Deaconess
American Academy of Dermatology Alice Gottlieb, MD, PhD
Clinical Professor,
Icahn School of Medicine at Mt Sinai
Chief Clinical Officer
Immunoglobulin National Society Amy Clarke, DNP, RN, IgCNChief Clinical Officer
Membership Association members serve as organizational representatives, while other members’ affiliations are provided for transparency and should not be interpreted as organizational endorsement.
STAKEHOLDER REVIEW COUNCIL
• Derek Burns PharmD, MBA, BCPS, BCSCP
• Cheryl Cherry DNP, RN, BS, CRNI
• L R ("Rad") Dillon
• Nancy Ellis MBA, MHA, CHE, CRHC, CRMS, CPMA
• Kristen Fleury IgCN, RN, FNP-C
• Derek Fox MSN, RN, VA-BC, CRNI, NEA-BC
• Christina ("Chrissy") Halligan
• Jody Huss
• Danielle Jenkins MBA, BSN, RN, CRNI
• Mike Kirkbride
• Melesa Leach
• Grey Louisos CPC
• Brittany MacQuarrie RN
• Pamela ("Pam") McIntyre CRNI, IgCN, OCN, VA-BC
• Tricia McKee
• Jessica Mestayer
• Nigina Mirazimova DNP, NP, CNS, OCN
• Wanda Kay North RN, CCRC
• Kathleen ("Katie") Ogle CPB, BS
• Ashley Pendergrass BCSCP, IgCP
• Barbara Petroff MS, RPh, BCSCP, FNHIA, FASHP, CAC
• Lauren Pica RN, Advance Practice Registered Nurse, FNP-C
• Shaylee Rowe APRN-CNP
• Marvin Siegel CRNI
• Reetu Singh AAPC, CPC
• Jacqueline ("Jacq") Sommer-Smith BSN, RN
• Tara Wright DNPc, BSN, RN, AVA- BC
• Rafe Young
Join Committees
Want to participate in the next edition of the NICA Standards of Excellence?
Seek Clarity
Looking for clarity on the intent of a Standard?
Submit Ideas
Have ideas for a new Standard or revision?
Want to help shape the next edition of the NICA Standards?
All stakeholders will have the chance to share feedback during the open review period—follow us on social media and subscribe to our newsletters to stay in the loop